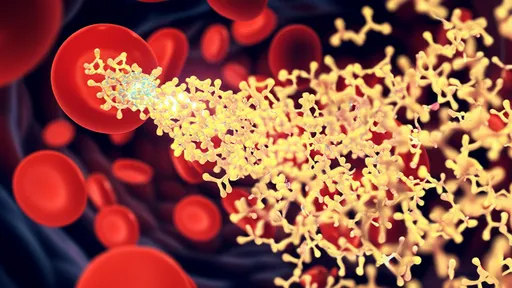
In the relentless pursuit of more effective cancer treatments, a class of therapeutics known as antibody-drug conjugates, or ADCs, has emerged as a transformative force. These sophisticated molecules, often described as "biological missiles" or "guided warheads," represent a paradigm shift in oncology. They are engineered with a singular, elegant purpose: to deliver potent cytotoxic agents directly to cancer cells while largely sparing healthy tissues. This targeted approach stands in stark contrast to traditional chemotherapy, which operates on a scorched-earth principle, attacking rapidly dividing cells throughout the body with devastating side effects. The journey of ADCs from a compelling theoretical concept to a cornerstone of modern oncology is a testament to decades of scientific perseverance and innovation.
The fundamental architecture of an ADC is a marvel of bioengineering, comprising three distinct components meticulously linked together. At its core is a monoclonal antibody, a protein exquisitely designed to recognize and bind to a specific antigen predominantly found on the surface of cancer cells. This antigen, such as HER2, TROP2, or CD33, serves as the unique "zip code" for the tumor. Attached to this antibody is the cytotoxic payload, a highly potent cell-killing drug, often a derivative of traditional chemotherapeutics but with a potency that would be far too toxic for systemic administration. Connecting these two elements is the chemical linker, a critical and sophisticated bridge that must remain stable in the bloodstream but efficiently release its deadly cargo once the ADC has been internalized by the cancer cell.
The mechanism of action is a precisely choreographed sequence of events. The process begins when the ADC circulates through the body, its antibody component seeking out its specific antigenic target. Upon finding and binding to the antigen on a cancer cell, the entire complex is ushered inside the cell through a process called receptor-mediated endocytosis. Once inside the acidic environment of the endosome or lysosome, the linker is cleaved, unleashing the potent payload. This cytotoxic agent then proceeds to wreak havoc on the cancer cell's vital machinery, typically by disrupting DNA replication or inhibiting microtubule function, leading to programmed cell death, or apoptosis. This "seek and destroy" mechanism maximizes tumor cell kill while minimizing the exposure of healthy cells to the toxic drug.
The clinical impact of ADCs has been nothing short of revolutionary, particularly in areas of high unmet medical need. In HER2-positive breast cancer, the advent of trastuzumab emtansine (T-DM1, Kadcyla®) and, more recently, fam-trastuzumab deruxtecan-nxki (Enhertu®) has dramatically altered the treatment landscape. These agents have provided powerful new options for patients whose disease has progressed on prior HER2-targeted therapies, offering significant improvements in survival. Similarly, in hematologic malignancies, ADCs like brentuximab vedotin (Adcetris®) for Hodgkin lymphoma and gemtuzumab ozogamicin (Mylotarg®) for acute myeloid leukemia have demonstrated profound efficacy, leading to their establishment as standard-of-care treatments in various settings.
Despite their promise, the development and application of ADCs are not without significant challenges. A primary hurdle is the identification of truly tumor-specific antigens. An ideal target is abundantly expressed on cancer cells but absent or minimally present on healthy tissues. The reality is that many targets are merely "tumor-associated," expressed at higher levels on cancer cells but still present on some normal cells, which can lead to on-target, off-tumor toxicity. Furthermore, the stability of the linker is paramount; premature release of the payload in the bloodstream can cause severe side effects, such as the neutropenia and thrombocytopenia commonly observed with some ADCs. Another growing concern is the emergence of resistance mechanisms, where tumors downregulate the target antigen or upregulate drug efflux pumps, rendering the ADC ineffective over time.
Looking toward the horizon, the future of ADC technology is blazing with innovation. The so-called "next-generation" ADCs are being engineered with features designed to overcome current limitations. Scientists are developing ADCs with bystander killing effects, where the released payload can diffuse into and kill neighboring cancer cells that may not express the target antigen, thereby addressing tumor heterogeneity. There is also a strong focus on creating ADCs with novel payloads that work through different mechanisms, such as inducing immunogenic cell death, which can help rally the patient's own immune system against the cancer. The exploration of bispecific antibodies as the targeting moiety, capable of binding two different antigens simultaneously, promises even greater tumor selectivity and potency.
The expansion of ADC applications is another exciting frontier. While their success in oncology is well-established, the fundamental technology of targeted delivery is now being explored in other therapeutic areas. In autoimmune diseases, ADCs are being designed to selectively deplete hyperactive immune cells. In infectious diseases, researchers are investigating ADCs that can deliver potent antibiotics directly to bacteria-infected cells. The potential to direct a therapeutic agent with such precision opens up a new realm of possibilities across medicine, suggesting that the impact of this technology will extend far beyond the field of cancer.
In conclusion, antibody-drug conjugates stand as a powerful validation of the targeted therapy paradigm. They embody a more intelligent, precise, and ultimately more humane approach to treating complex diseases like cancer. By marrying the specificity of antibodies with the potency of cytotoxic drugs, they have successfully expanded the therapeutic boundary for numerous malignancies once considered untreatable. The journey has been long and fraught with challenges, but the clinical successes have unequivocally proven the value of this approach. As research continues to refine their design, expand their targets, and unlock new applications, ADCs are poised to remain at the forefront of biomedical innovation, offering renewed hope and extended lives to patients around the world.

By /Oct 21, 2025

By /Oct 21, 2025

By Emily Johnson/Oct 21, 2025

By /Oct 21, 2025

By /Oct 21, 2025

By /Oct 21, 2025

By /Oct 21, 2025

By /Oct 21, 2025

By /Oct 21, 2025

By /Oct 21, 2025
By /Oct 21, 2025

By /Oct 21, 2025

By /Oct 21, 2025

By Natalie Campbell/Oct 21, 2025

By /Oct 21, 2025

By /Oct 21, 2025

By Noah Bell/Oct 21, 2025

By /Oct 21, 2025
By /Oct 21, 2025